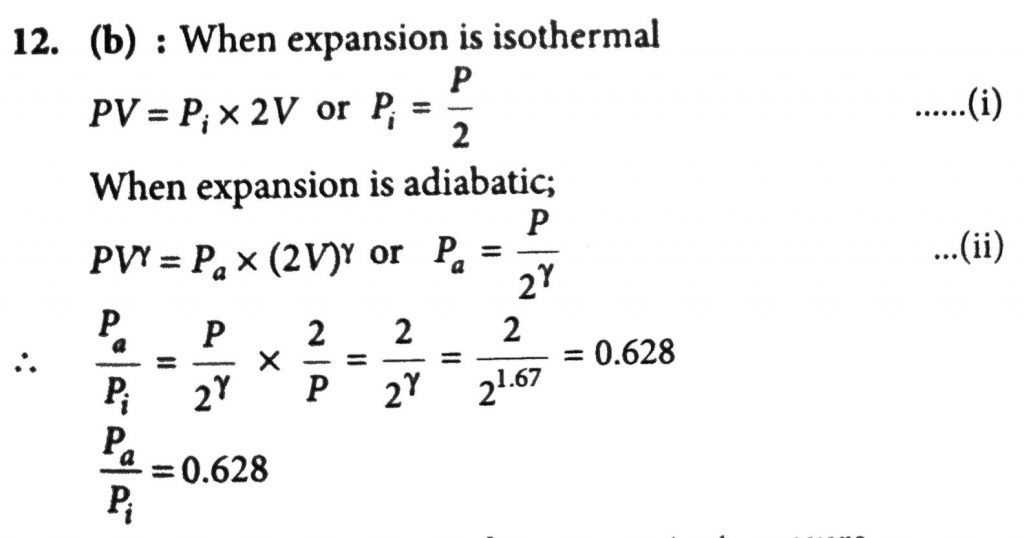
An ideal gas with pressure P, volume V and temperature T is expanded isothermally to a volume 2V and a final pressure P_i, If the same gas is expanded adiabatically to a volume 2V, the final pressure P_a. The ratio of the specific heats of the gas is 1.67. The ratio (P_a)/(P_1) is