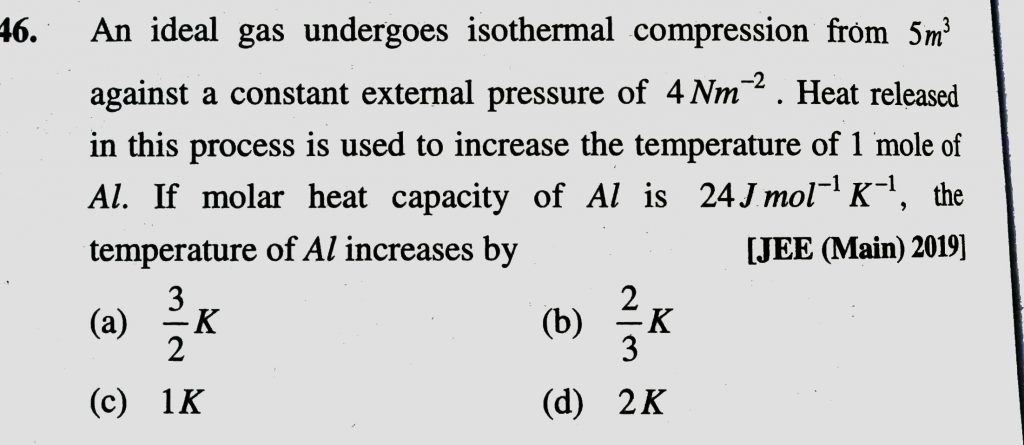
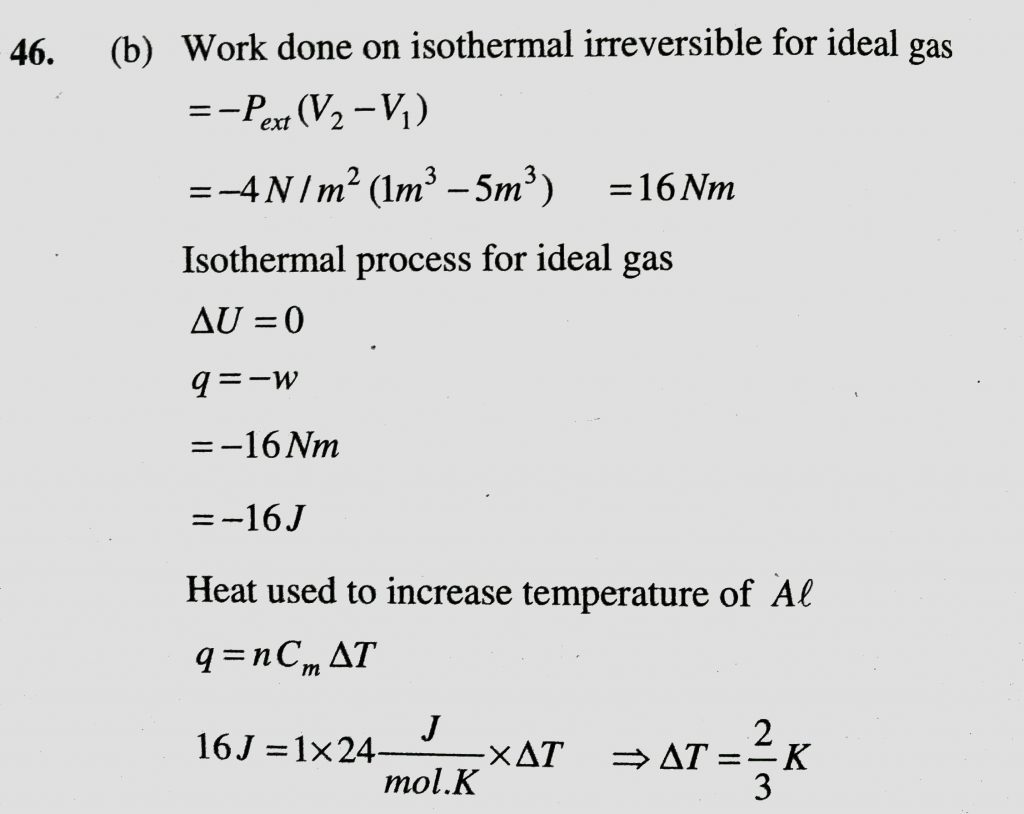
An ideal gas undergoes isothermal compression from 5 m ^ 3 to 1 m^3 against a constant external pressure of 4Nm −2 . Heat released in this process is used to increase the temperature of 1mole of Al. If molar heat capacity of Al is 24 J/mol/K , the temperature of Al increased by