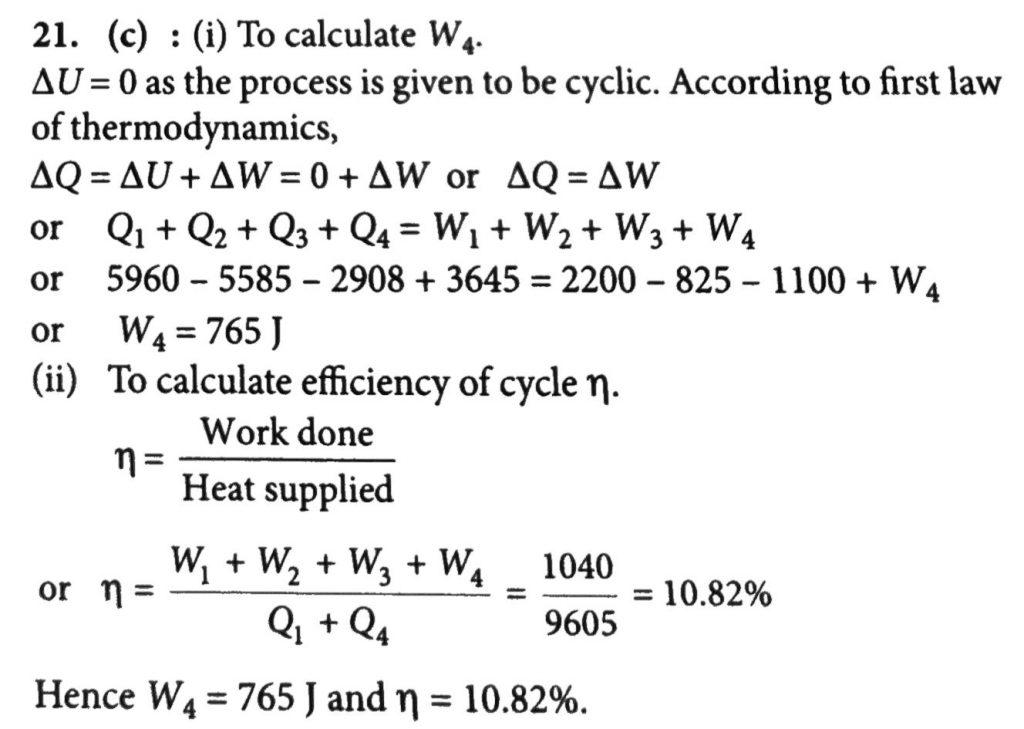
An ideal gas is taken a cyclic thermodynamic process through four steps. The amount of heat involved in these steps are Q1= 5960 J,Q2 =-5585J,Q3 =-2980 j and Q4 =3645J respectively. The corresponding works involved are W1= 2200 J, W2= -825J, W3 = -1100 J and W4 respectively.