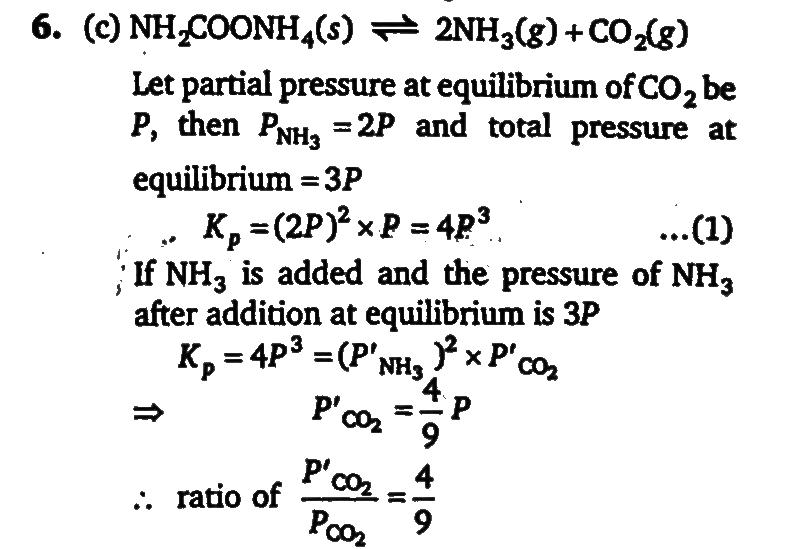Ammonium carbamate dissociates as NH2 COONH4(s) ⇋2NH 3(g) +CO 2(g) Ina closed vessel containing ammonium carbamate in equilibrium, ammonia is added such that the partial pressure of NH3 now equals to the original total pressure. calculate the ratio of total pressure of CO2 now to the original partial pressure CO2.