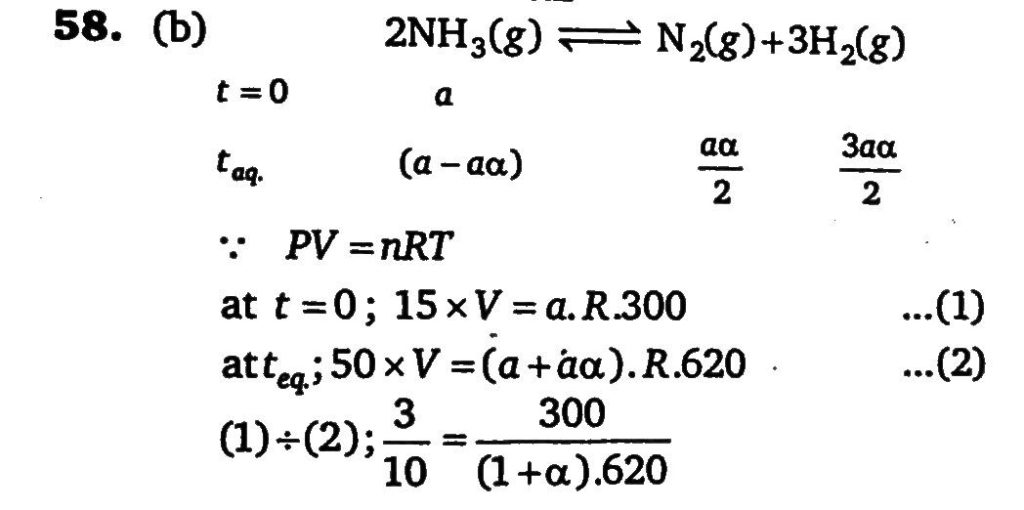Ammonia under pressure of 15 atm at 27^oC is heated to 347^oC in a closed vessel in the presence of catalyst. Under the conditions, NH 3 is partially decomposed according to the equation, 2NH3 ⇌ N2 +3H2 . The vessel is such that the volume remains effectively constant whereas pressure increases to 50 atm. Calculate the percentage of NH3 actually decomposed.