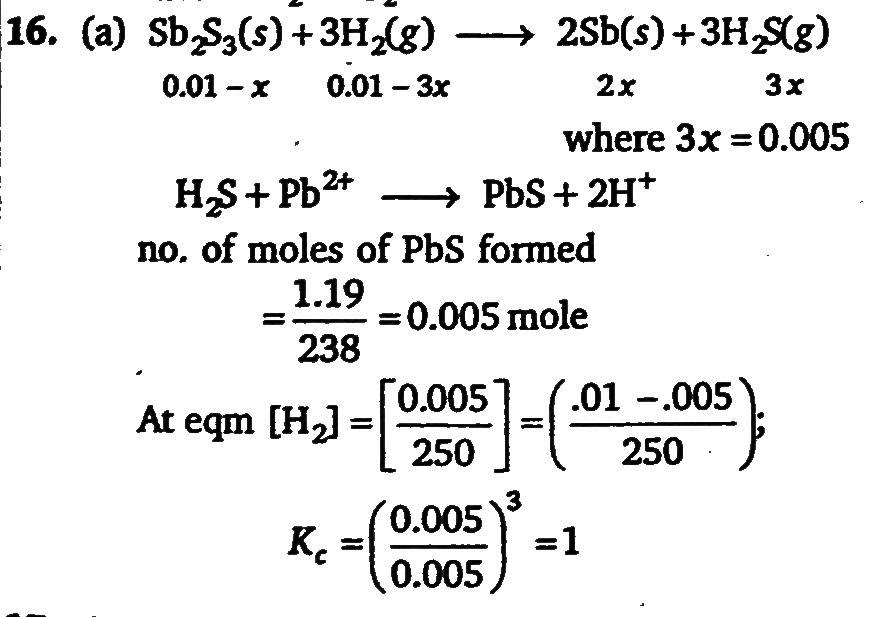
A vessel of 250 litre was filled with 0.01 mole of Sb2S3 and 0.01 mole of H2 to attain the equilibrium at 440^∘C as Sb2S3(s)3H2(g)⇔2Sb(s)+3H2D(g) After equilibrium, the H2S formed was analysed was analysed by dissolved it in water and treating with excess of Pb20+ to give 1.19 g of PbS as precipitate. What is the value of Kc at 440∘C ?