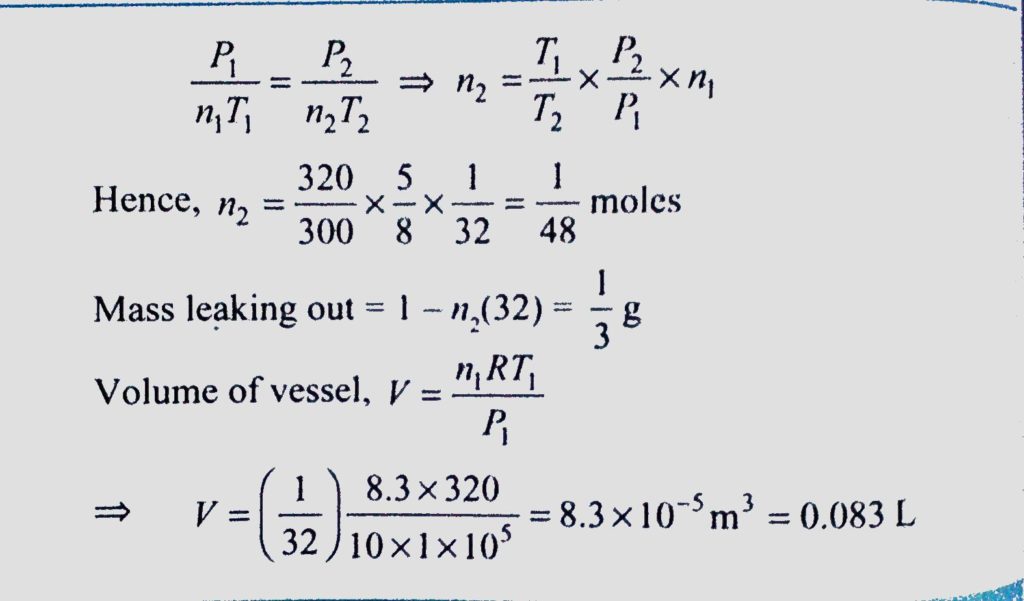A vessel containing 1g of oxygen at a pressure of 10atm and a temperature of 47C. It is found that because of a leak, the pressure drops to 5/8th of its original value and the temperature falls to 27C. Find the volume V of the vessel and the mass m of oxygen that is leaked out in litres.