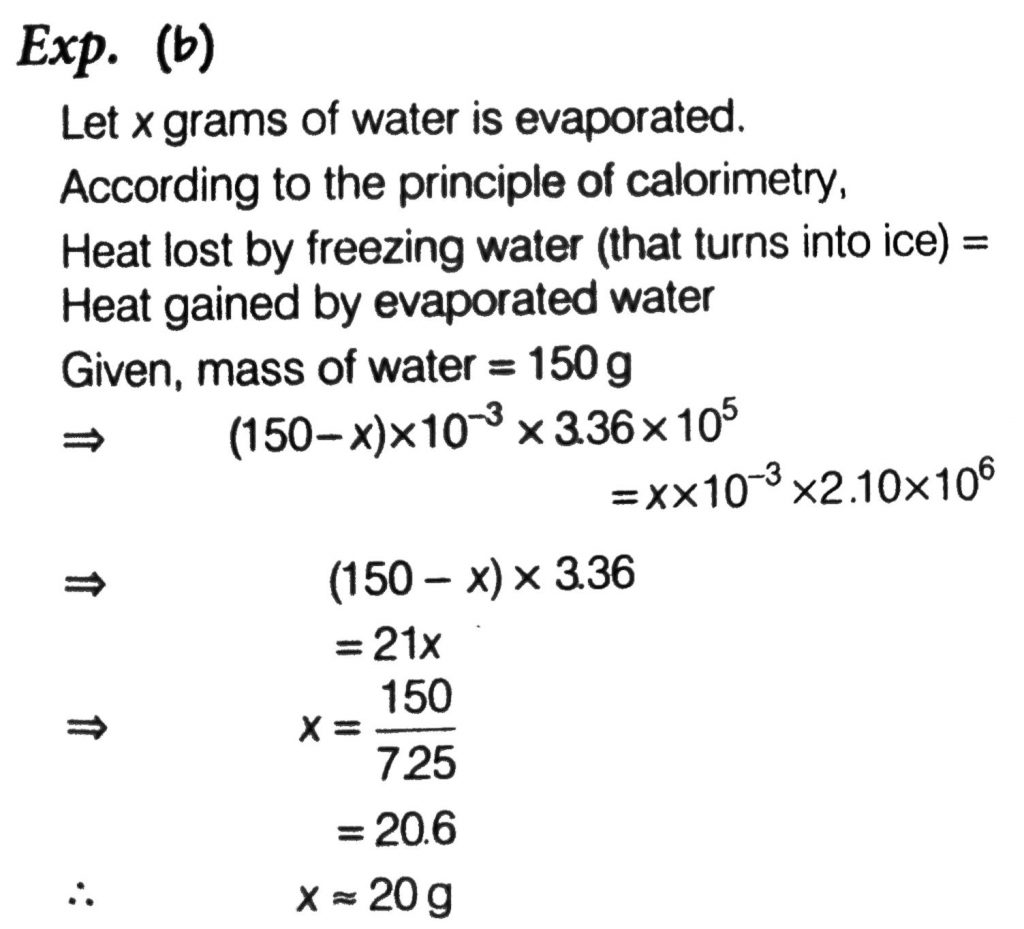
A thermally insulated vessel contains 150g of water at 0 C. Then the air from the vessel is pumped out adiabatically. A fraction of water turns into ice and the rest evaporates at 0 C itself. The mass of evaporated water will be closest to: (Latent heat of vaporization of water = 2.10×10^6 J kg^−1 and Latent heat of Fusion of water =3.36×10^5 J kg^−1 )