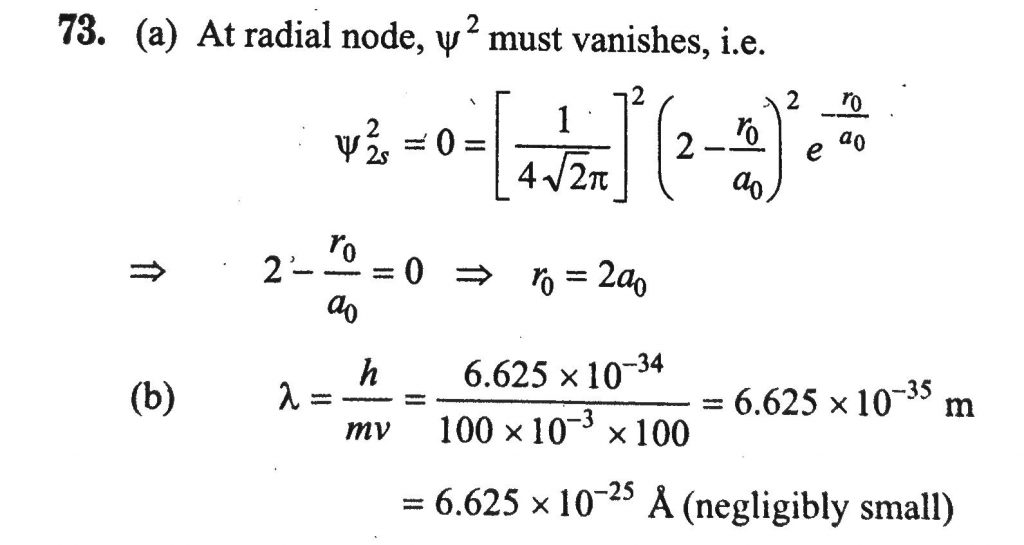(a) The Schrodinger wave equation for hydrogen atom is: Ψ2s = 1/ 4 (2 π)^v2 (1/a0)3/2 [2− a0/r ]e^−r/a 0 where a 0 is Bohr radius. If the radial node in 2s be at r0 , then find r in terms of a0 . (b) A ball of 100 g mass is thrown with a velocity of 100 m/s. Find out the value of wavelength of base ball.