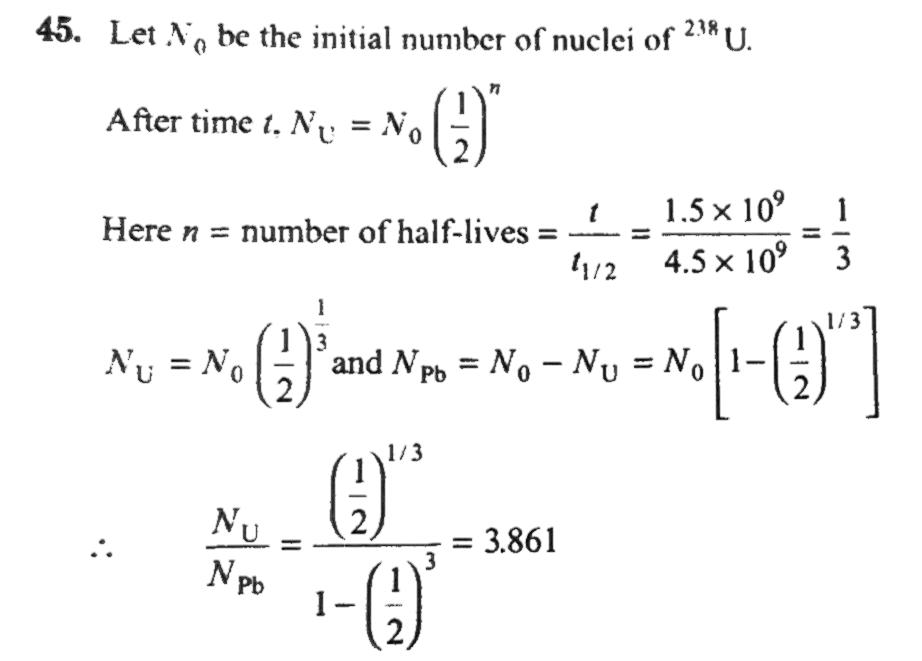A rock is 1.5 * 10^9 years old. The rock contains 238 U which disintegrates to form 206 Pb. Assume that there was no 206 Pb in the rock initially and it is the only stable product formed by the decay. Calculate the ratio of number of nuclei of .^(238)U to that of .^(206)Pb in the rock. Half-life of .^(238)U is 4.5 x 10^(9). years. (2^(1/3)=1.259) .