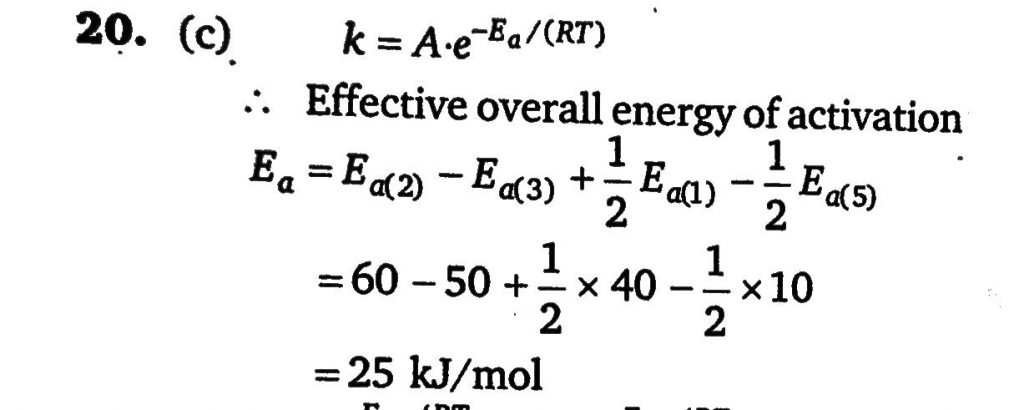A reaction takes place in various steps. The rate constant for first, second, third and fifth steps are k1,k2,k3 and k5 respectively The overall rate constant is given by k=k2/k3(k1/k5)1/2 If activation energy are 40, 60, 50, and 10 kJ/mol respectively, the overall energy of activation (kJ/mol) is :