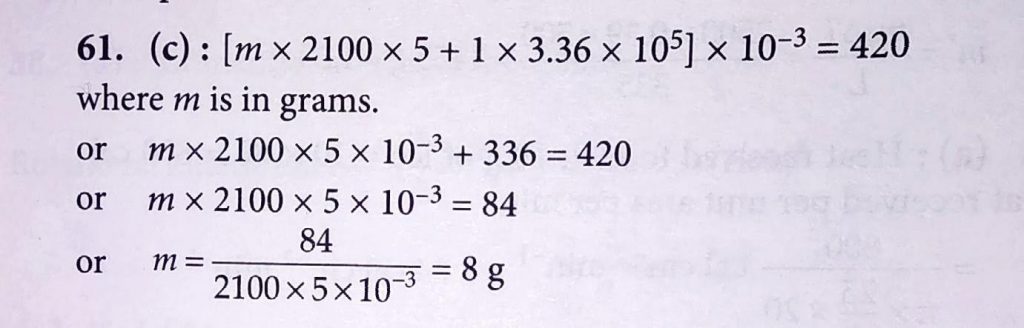A piece of ice (heat capacity =2100J kg^−1 ∘C^−1 and latent heat =3.36×10^5Jkg^−1) of mass m grams is at −5∘C at atmospheric pressure. It is given 420 J of heat so that the ice starts melting. Finally when the ice . Water mixture is in equilibrium, it is found that 1 gm of ice has melted. Assuming there is no other heat exchange in the process, the value of m is