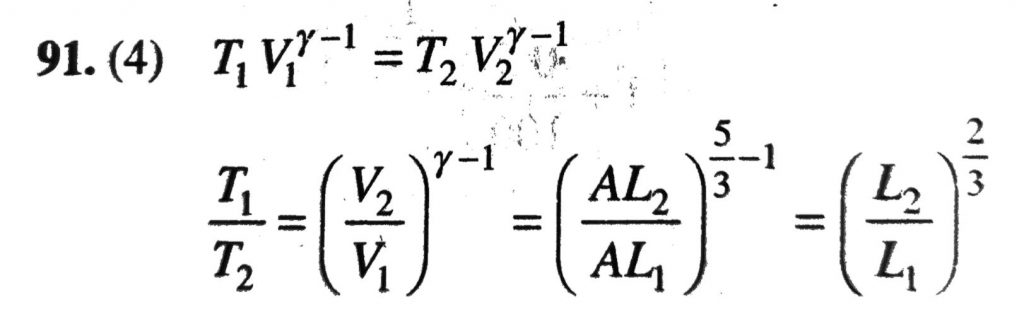A monoatomic ideal gas, initially at temperature T1 is enclosed in a cylinders fitted with a frictionless piston. The gas is allowed to expand adiabatically to a temperature T2 by releasing the piston suddenly If L1 and L2 the lengths of the gas column before and after expansion respectively, then then (T1 / T2) is given by