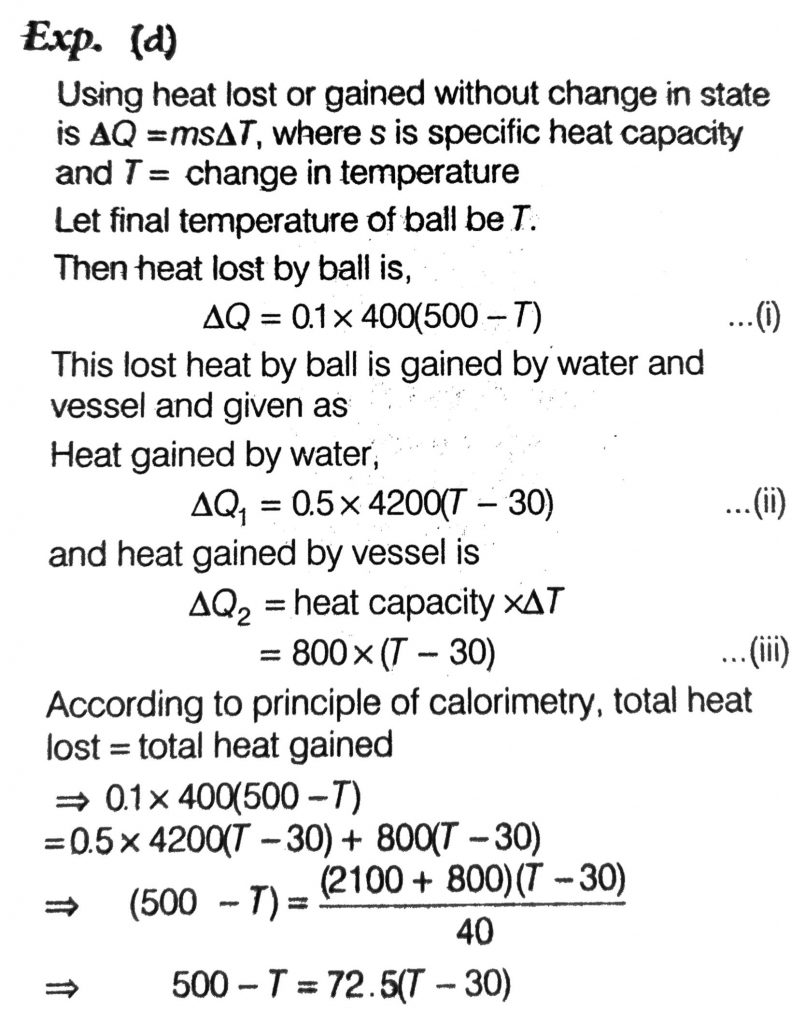
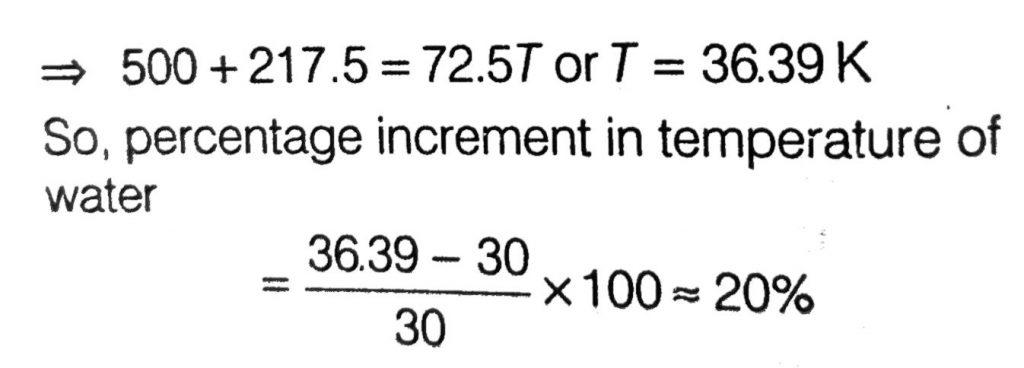A metal ball of mass 0.1 kg is heated upto 500ºC and dropped into a vessel of heat capacity 800 JK^–1 and containing 0.5 kg water. The initial temperature of water and vessel is 30ºC. What is the approximate percentage increment in the temperature of the water? [Specific Heat Capacities of water and metal are, respectively, 4200 Jkg–1 K–1 and 400 Jkg–1 K–1]