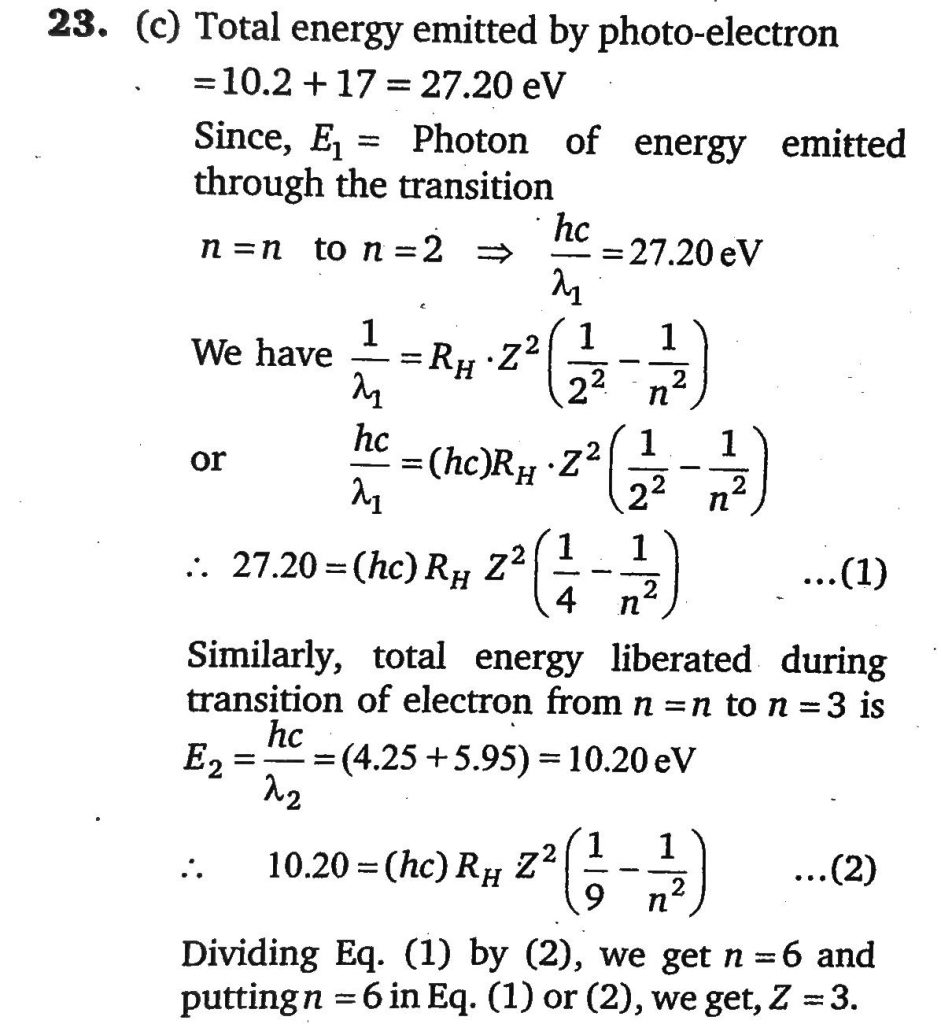A hydrogen like species (atomic number Z) is present in a higher excited state of quantum number n. This excited atom can make a transition to the first excited state by successive emission of two photons of energies 10.20eV and 17.0eV respectively. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4.25eV and 5.95eV respectively. Determine the value of Z.