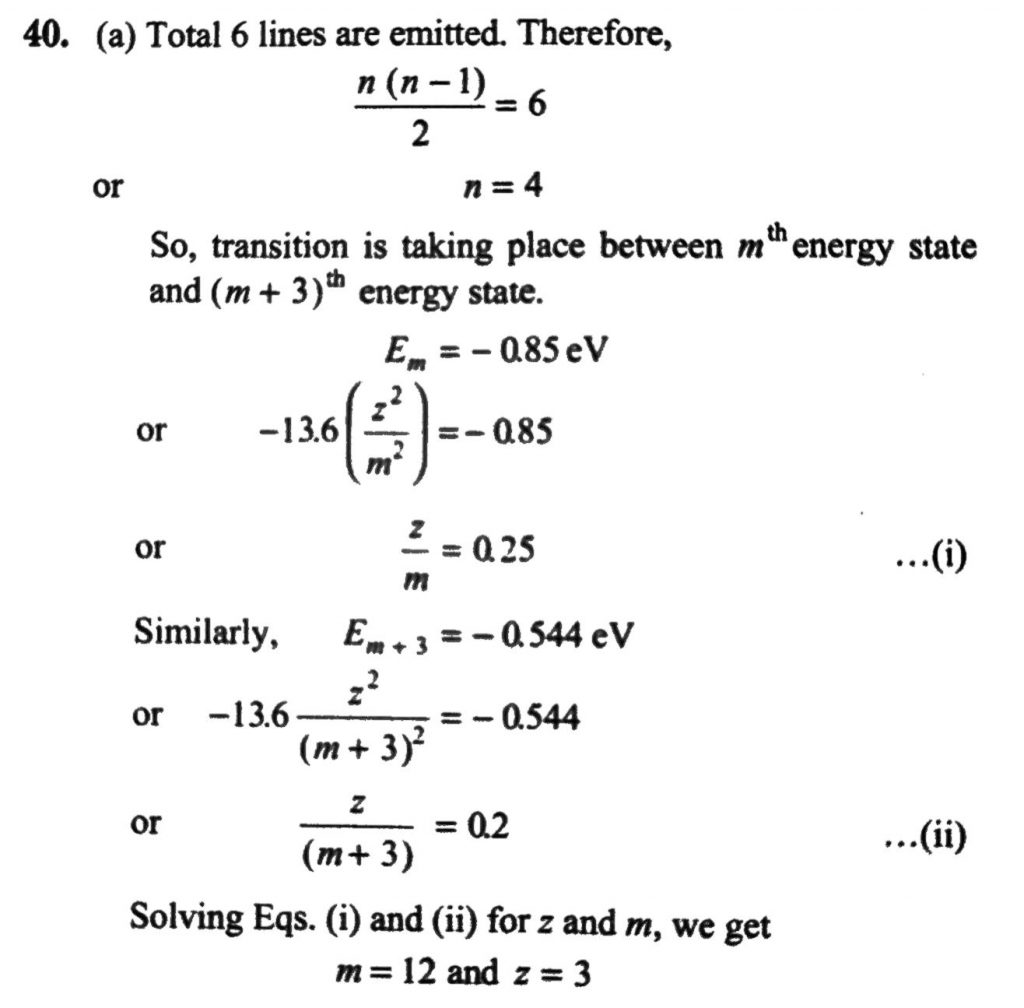
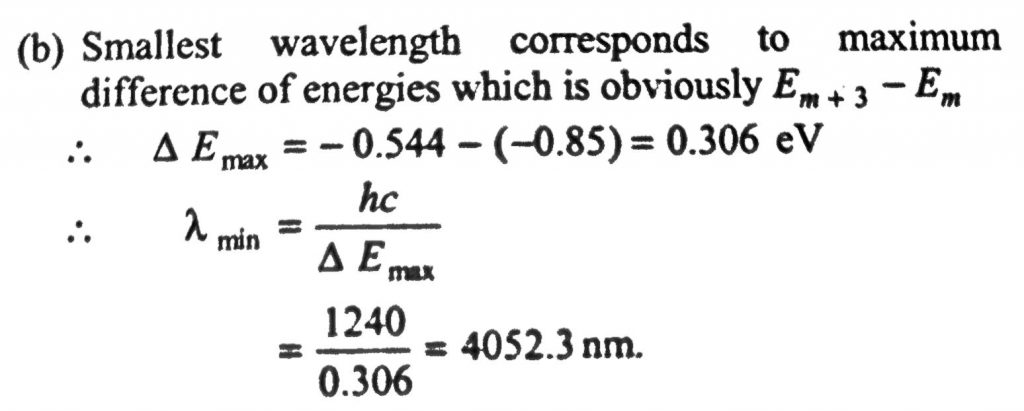
A hydrogen-like atom (described by the Bohr model) is observed to emit six wavelengths, originating from all possible transitions between a group of levels. These levels have energies between –0.85 eV and –0.544 eV (including both these values). (a) Find the atomic number of the atom. (b) Calculate the smallest wavelength emitted in these transitions. (Take hc = 1240 eV.nm. Ground state energy of hydrogen atom = – 13.6 eV)