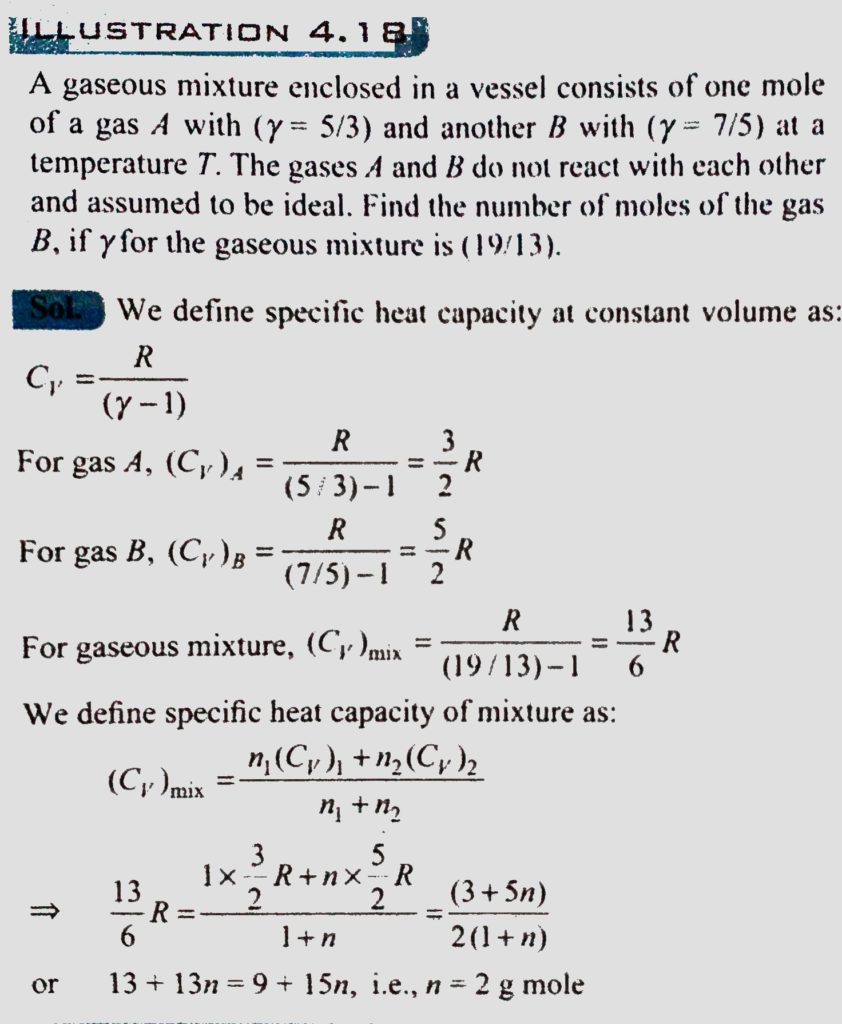
A gaseous mixture enclosed in a vessel consists of one mole of a gas A with γ=(5/3) and some amount of gas B with γ=7/5 at a temperature T. The gases A and B do not react with each other and are assumed to be ideal. Find the number of gram moles of the gas B if γ for the gaseous mixture is