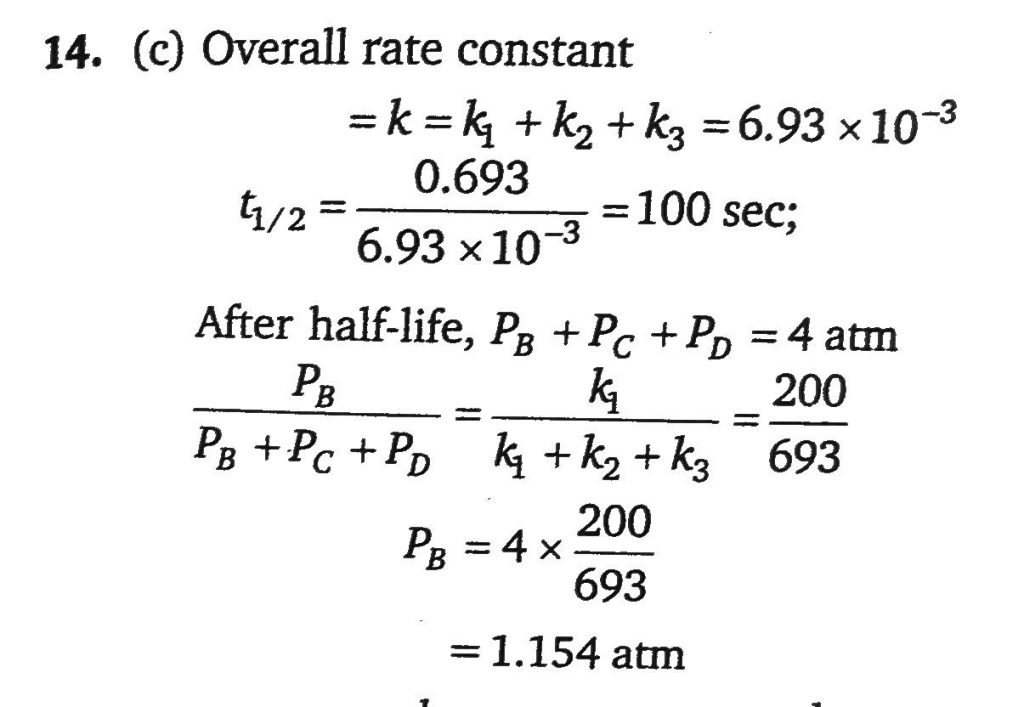A gaseous compound A reacts by three independent first order process (as shown in figure) with rate constant 2×10^−3,3×10^−3 and 1.93×10^−3sec^−1 for products B, C and D respectively, If initially pure A was taken in a closed container with p=8 atm then the partial pressure of B (in atm) after 100 sec from starting the experiment.