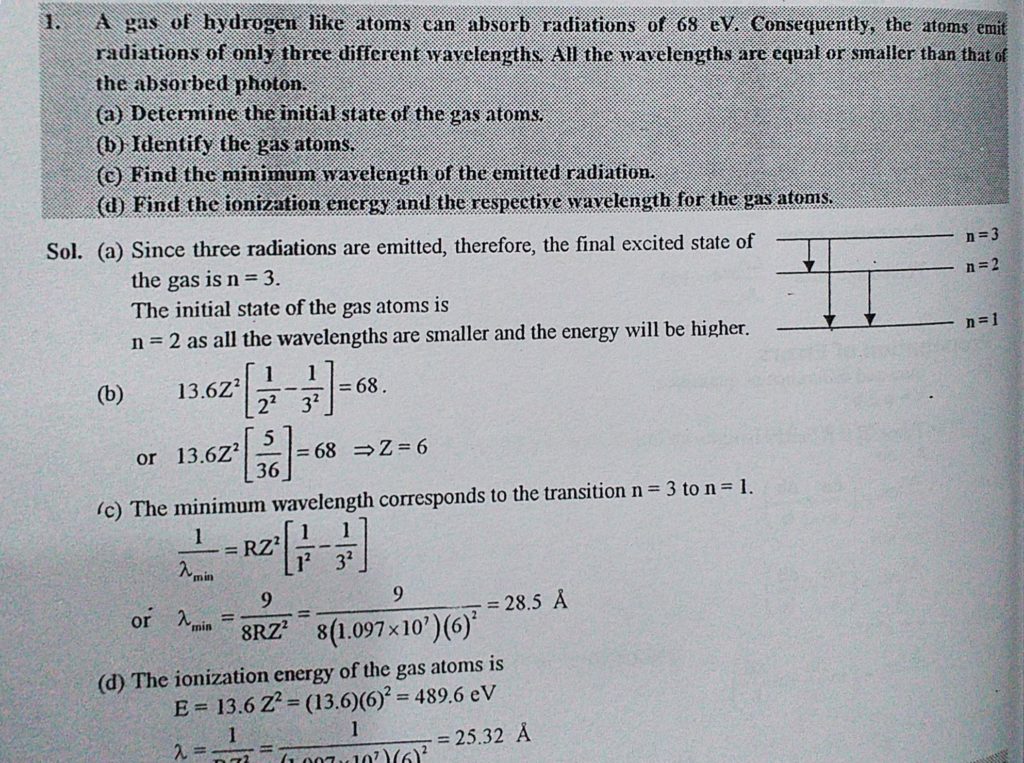
A gas hydrogen like atoms can absorb radiations of 68eV. Consequently, the atoms emit radiations of only three different wavelengths. All the wavelengths are equal or smaller than that of the absorbed photon. (a) Determine the initial state of the gas atoms. (b) Identify the gas atoms. (c) Find the minimum wavelength of the emitted radiation. (d) Find the ionization energy and the respective wavelength for the gas atoms.