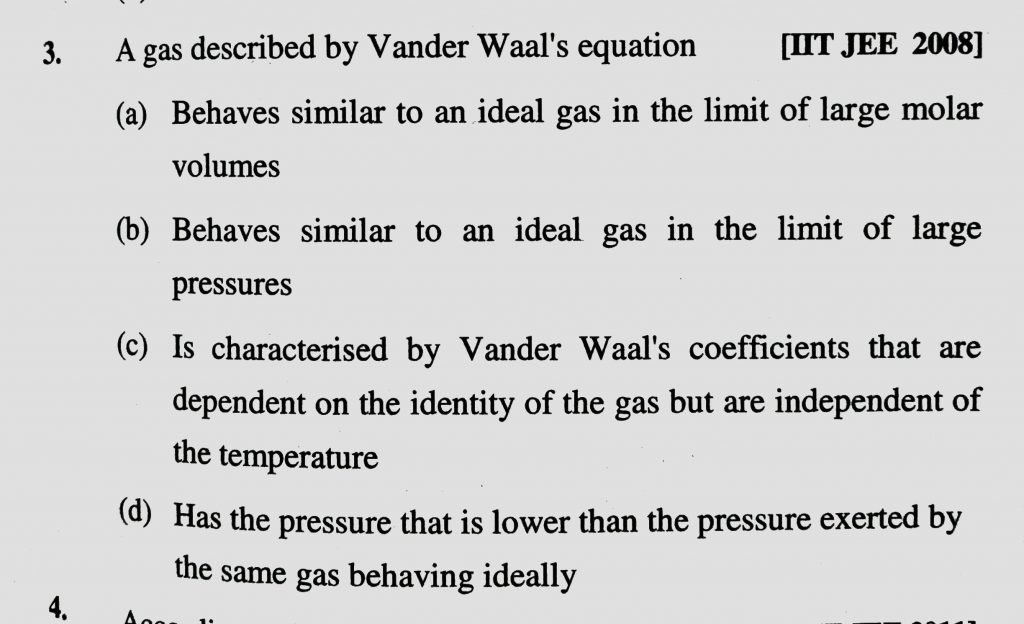
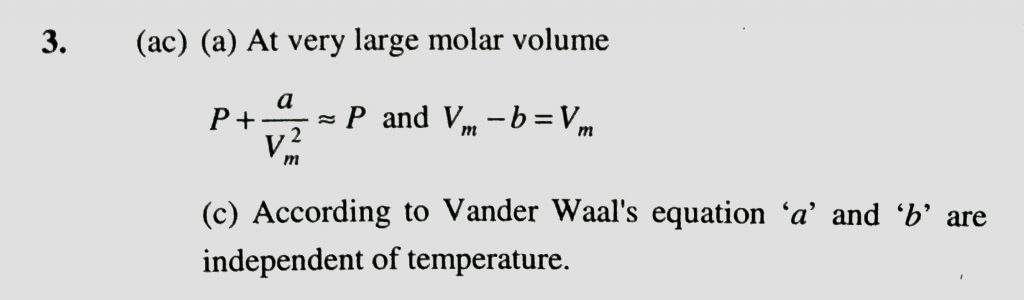
A gas described by Vander Waal’s equation (a) Behaves similar to an ideal in the limit of large molar volumes (b) Behaves similar to an ideal in the limit of large molar pressures (c) Is charcterised by Vander Waal’s coefficients that are dependent on the identify of the gas but are independent of the temperature (d) Has the pressure that is lower than the pressure exerted by the same gas behaving idealy