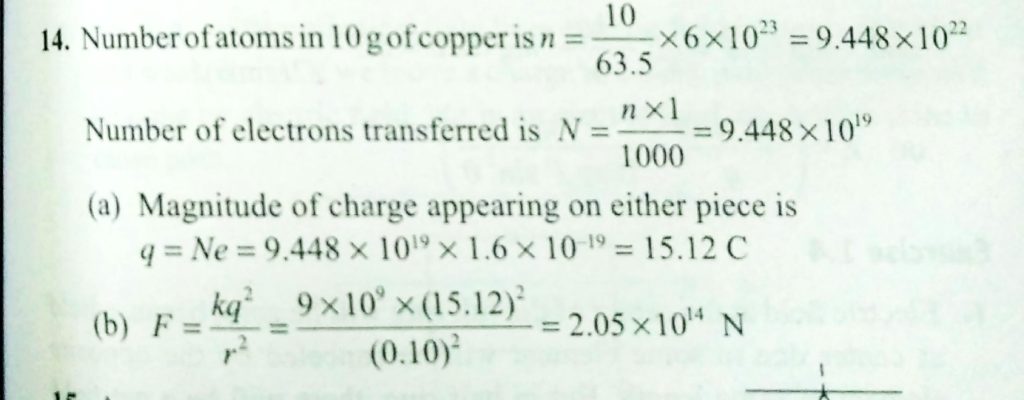A copper atom consists of copper nucleus surrounded by 29 electrons. The atomic weight of copper is 63.5 g mole-. Let us now take two pieces of copper each weighing 10 g. Let us transfer one electron from one piece to another for every 100 atoms in that piece. What will be the coulomb force between the two pieces after the transfer of electrons, if they are 1 cm apart? Given, Avogadro number = 6 x 10^23 mole-1,