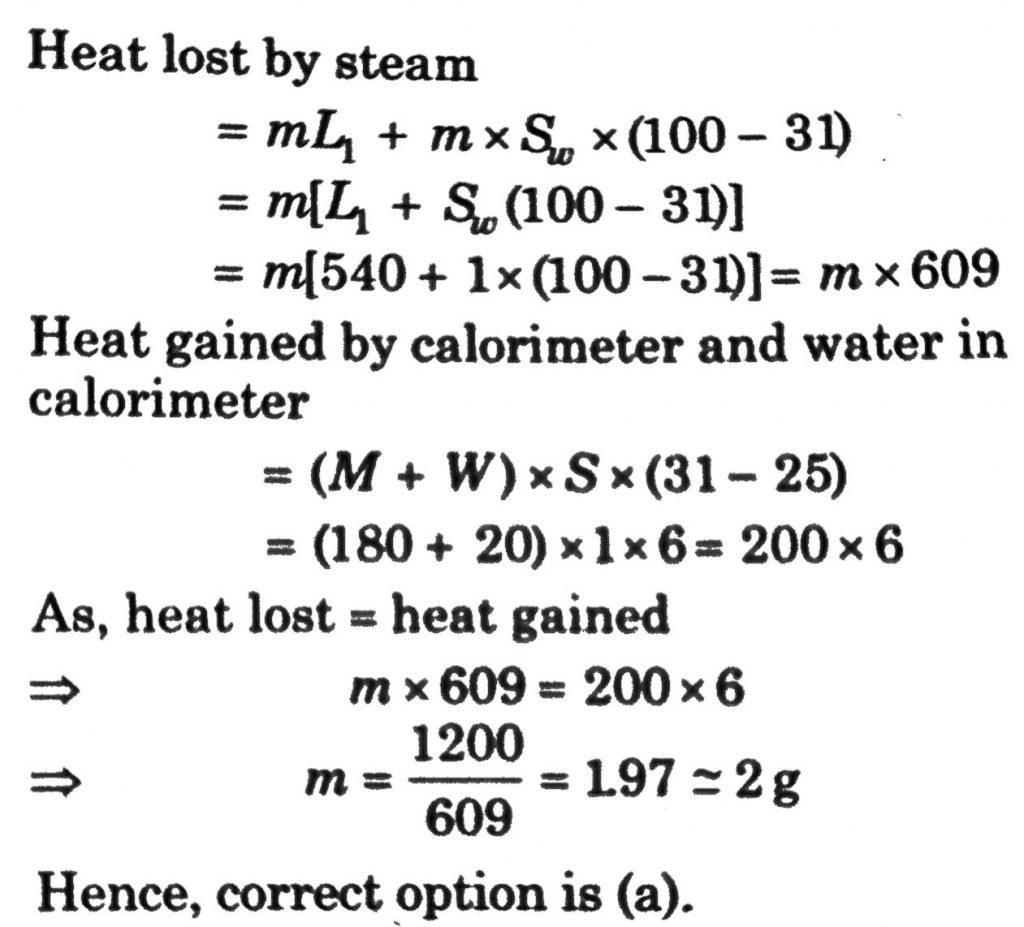
A calorimeter of water equivalent 20 g contains 180 g of water at 25°C. ‘m’ grams of steam at 100°C is mixed in it till the temperature of the mixture is 31°C. The value of ‘m’ is close to (Latent heat of water = 540 cal g^–1, specific heat of water = 1 cal g^–1 °C^–1)