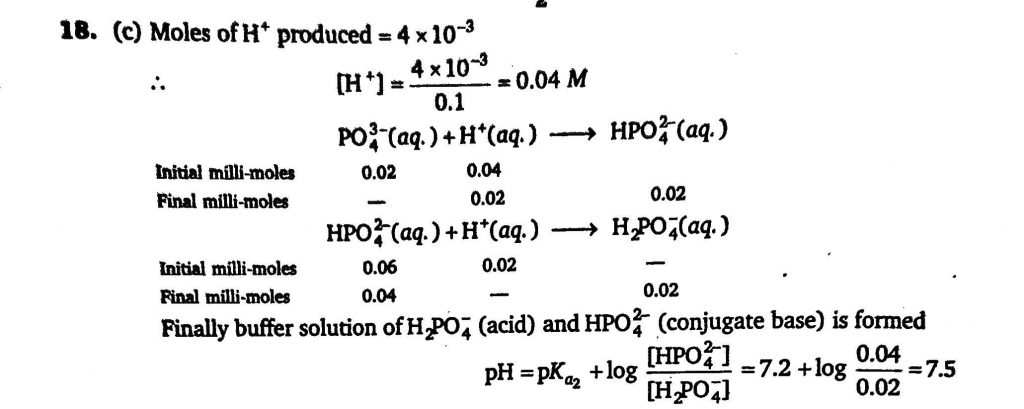
A buffer solution 0.04 M in Na2HPO4 and 0.02 in Na3PO4 is prepared. The electrolytic oxidation of 1.0 milli-mole of the organic compound RNHOH is carried out in 100 mL of the buffer. The reaction is RNHOH + H2O→RNO2+4H^+ +4e− The approximate pH of solution after the oxidation is complete is : [Given:forH3PO4,pKa1=2.2,pKa2=7.20,pKa3=12]