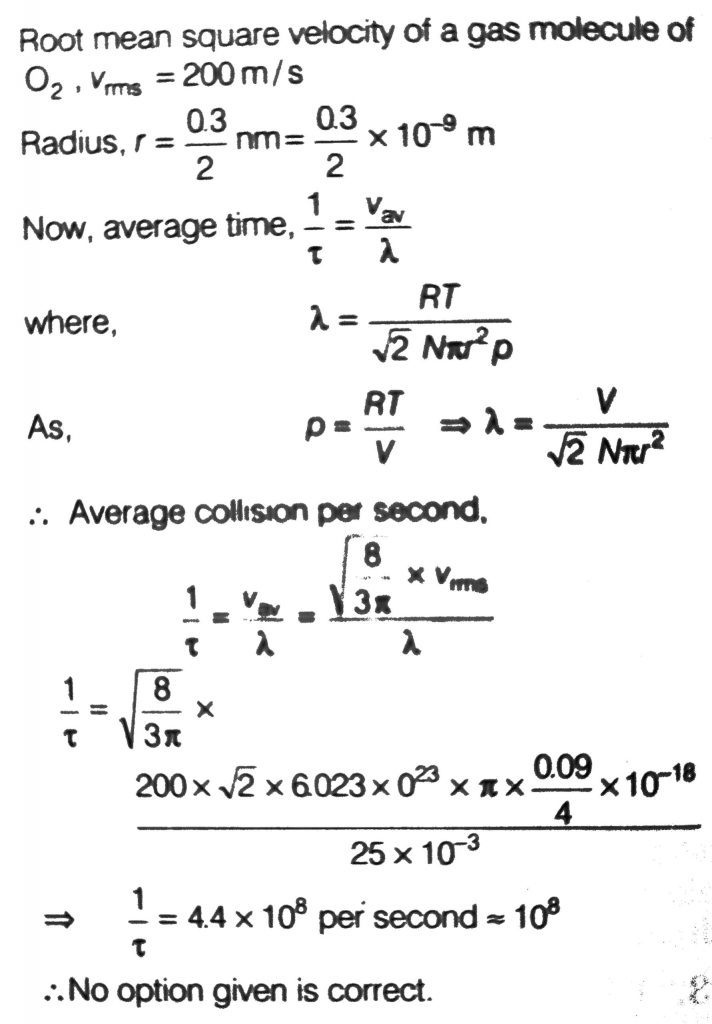
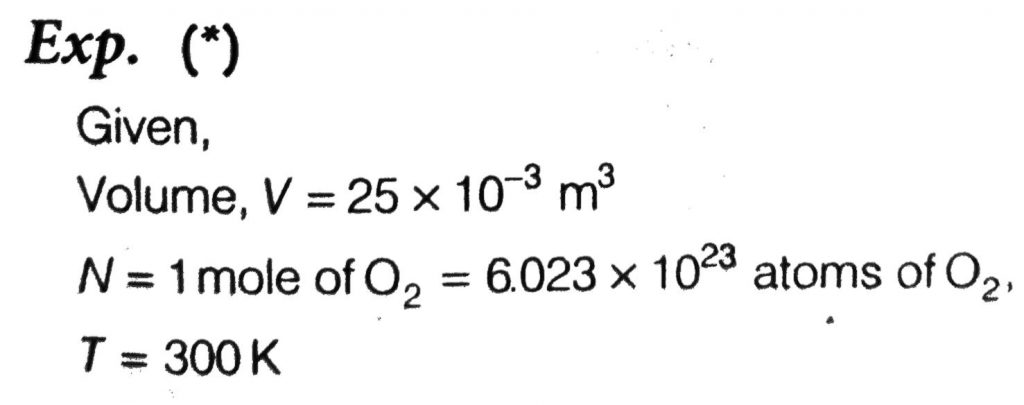A 25 × 10^–3 m 3 volume cylinder is filled with 1 mol of O2 gas at room temperature (300 K). The molecular diameter of O2, and its root mean square speed, are found to be 0.3 nm and 200 m/s, respectively. What is the average collision rate (per second) for an O2 molecule ?