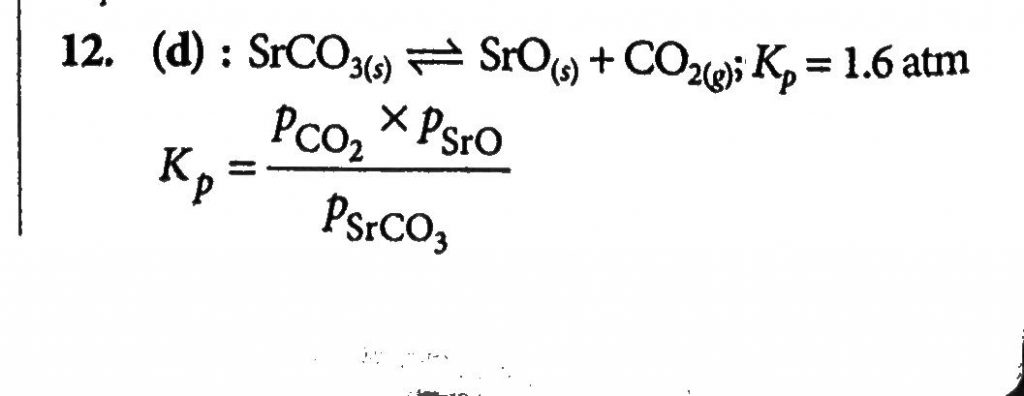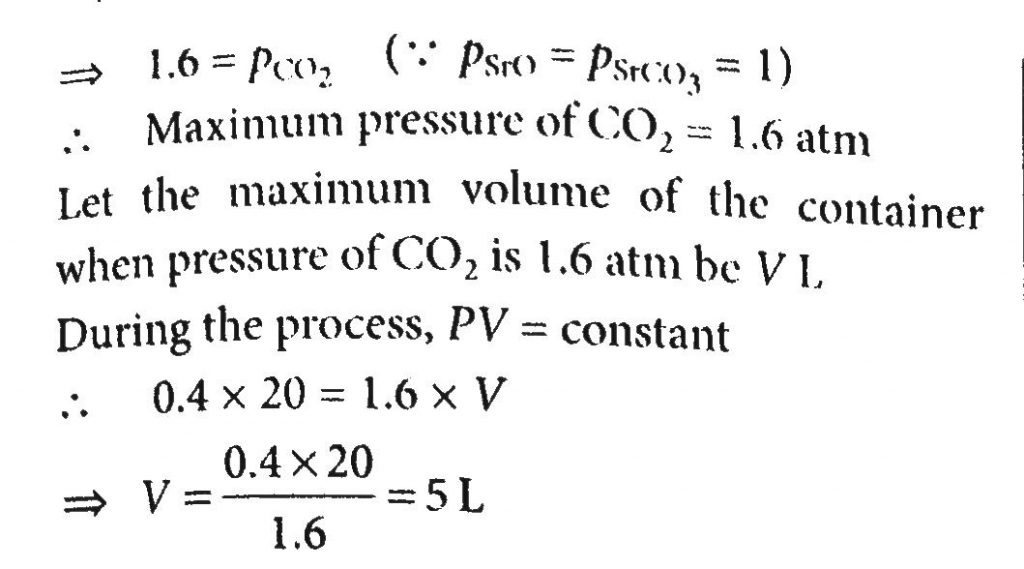A 20-liter container at 400K contains CO 2(g)at pressure 0.4 atm and an excess of SrO. The volume of the container is now decreased by moving the movable piston fitted in the container. The maximum volume of the container, when the pressure of CO 2 attains its maximum value when the pressure of CO 2 attains its maximum value, will be:Given that: SrCO 3 (s)⇌SrO(s)+CO 2 (g), Kp=1.6 atm.