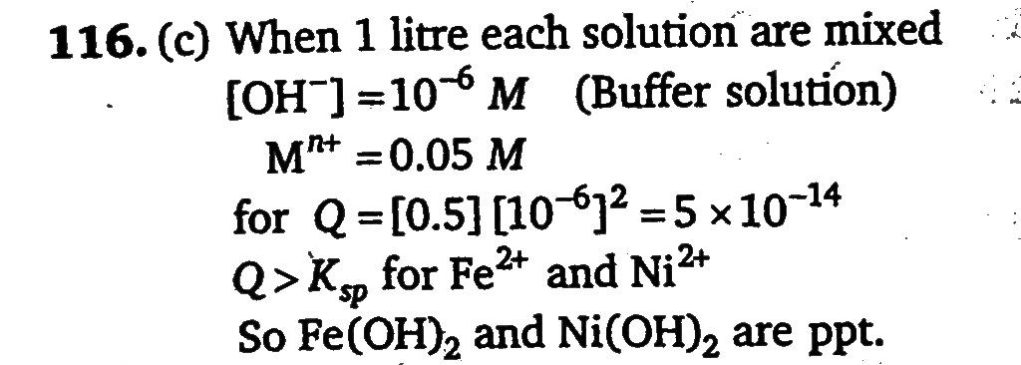A 1 litre solution containing NH4Cl and NH4OH has hydroxide ion ion concentration of 10^−6) mol/litre. Which of the following hydroxides could be precipitated when the solution is added to 1 litre solution of 0.1 M metal ions? (I) Ba(OH)2(Ksp=5×10^−3) , (II) Ni(OH)2(Ksp=1.6×10^−16) (III) Mn(OH)2(Ksp=2×10^−13) , (IV) Fe(OH)2(Ksp=8×10^−16)