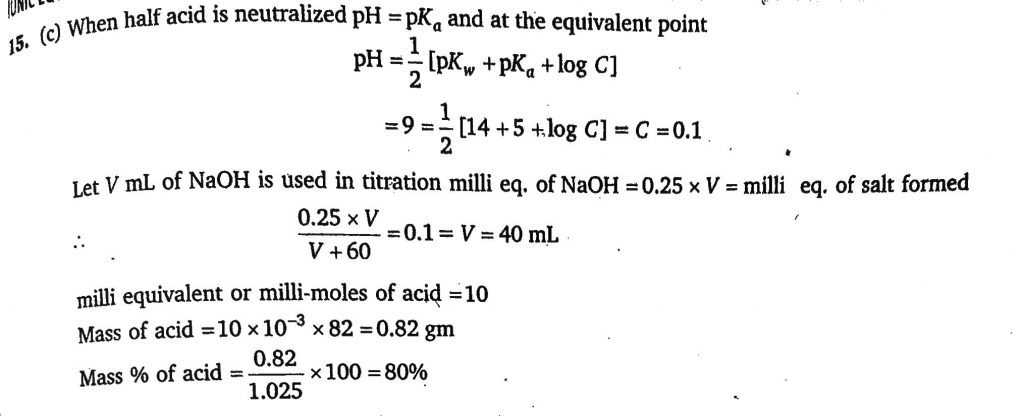
A 1.025 g sample containing a weak acid HX (mol. Mass=82) is dissolved in 60 mL. water and titrated with 0.25 M NaOH. When half of the acid was neutralised the pH was found to be 5.0 and at the equivalence point the pH is 9.0. Calculate mass percentage of HX in sample :