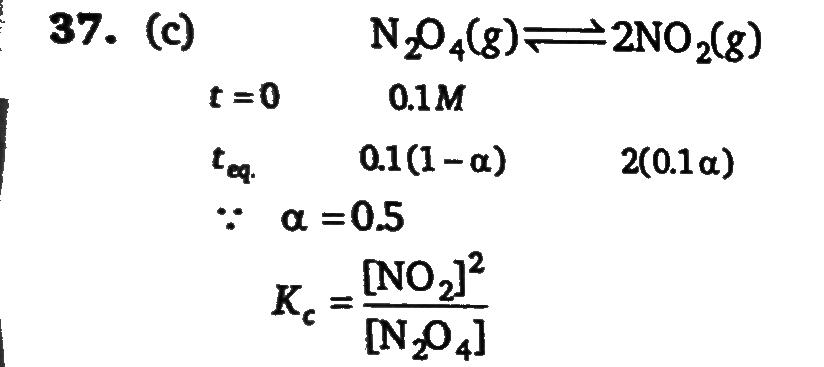9.2 grams of N2O4(g) is taken in a closed one lite vessel and heated till the following equilibrium is reached N2O4(f) ⇌ 2NO2(g). At equilibrium , 50% N2O4(g) is dissociated. What is the equilibrium constant (in mol litre) (molecular weight of N2 O4 = 92)