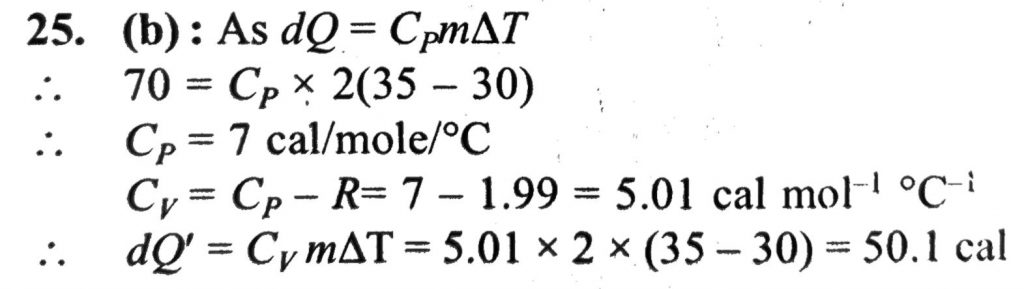70 calorie of heat are required to raise the temperature of 2 mole of an ideal gas at constant pressure from 30°C to 35°C. The amount of heat required to raise the temperature of the same sample of the gas through the same range at constant volume is