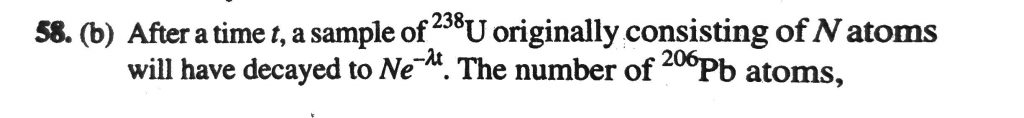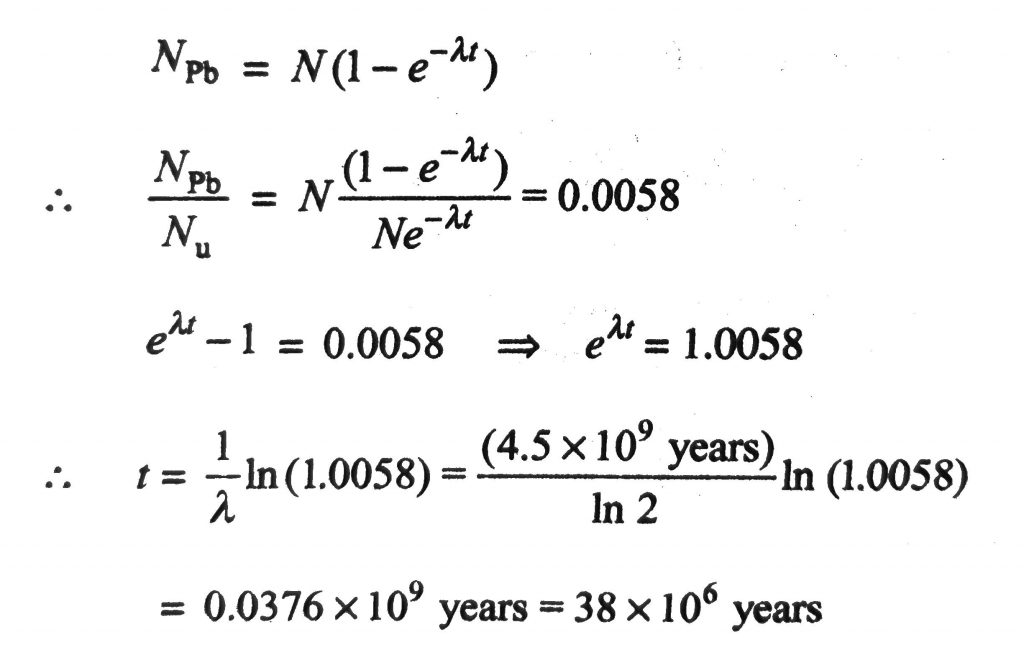238U decays with a half-life of 4.5×10^9 years, the decay series eventually ending at .206Pb, which is stable. A rock sample analysis shows that the ratio of the number of atoms of .206Pb to .238U is 0.0058. Assuming that all the .206Pb is produced by the decay of .238U and that all other half-lives on the chain are negligible, the age of the rock sample is (In1.0058 = 5.78 × 10^−3).