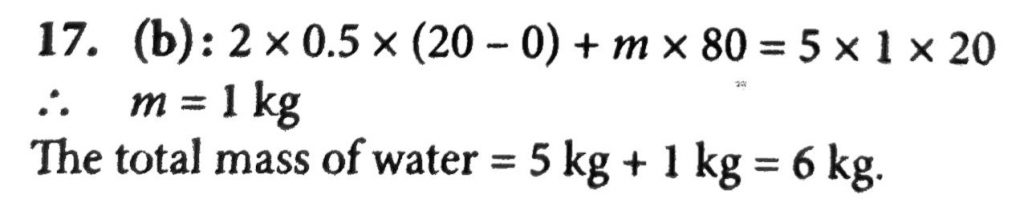2 kg of Ice at – 20° C is mixed with 5 kg of water at 20°C in an insulating vessel having a negligible heat capacity calculate the final mass of water remaining in the container. It is given that the specific heats of water and ice care 1 Kcal/kg per °C and 0.5 Kcal/kg 1°C while the latent heat of fusion of ice is 80 Kcal/kg.