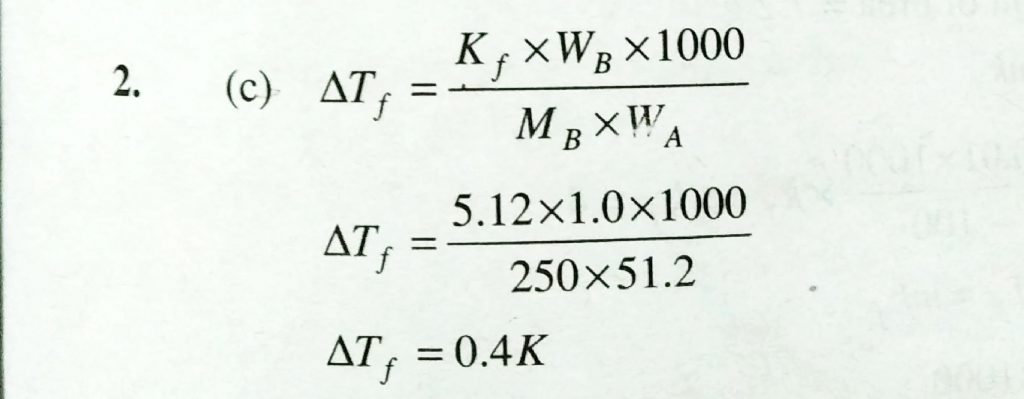100 g of a non – electrolyte solute ( molar 250 g /mol was dissolved in 51.2 g of benzene. If the freezing point depression constant, Kf of benzene is 5.12 kg /mol, the freezing point of benzene will be lowered by (a) 0.5 K (b) 0.2 K (c) 0.4 K (d) 0.3 K