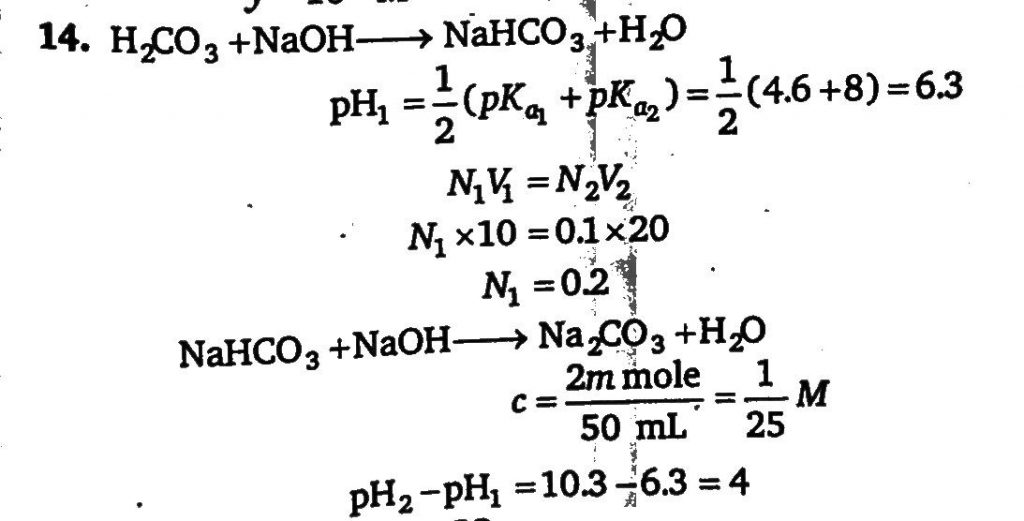10 mL of H2A(weak diprotic acid) solution is titrated against 0.1M NaOH. pH of the solution is plotted against volume of strong base added and following observation is made. If pH of the solution at first equivalence point is pH1 and at second equivalence point is pH2.Calculate the value of (pH_(2)-pH_(1))at25^ degree C Given: for H_(2)A,pK_(a_1)=4.6 and pK_(a_2)` =8, log 25=1.4