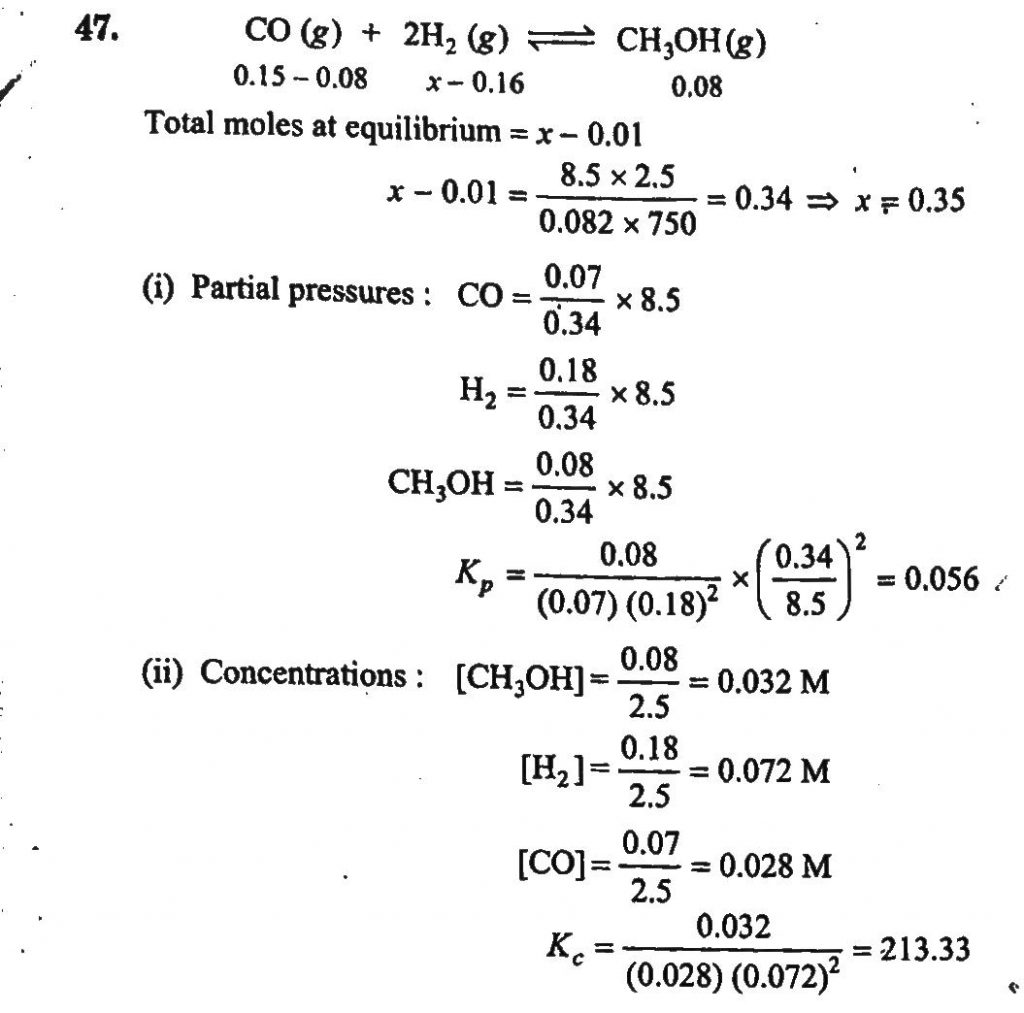
0.15 mole of CO taken in a 2.5L flask is maintained at 750K along with a catalyst so that the following reaction can take place: CO(g)+2H2(g)⇔CH3OH(g) Hydrogen is introduced until the total pressure of the system is 8.5 atm at equilibrium and 0.08 mole of methanol is formed. Calculate (i) Kp and Kc and (ii) the final pressure if the same amount of CO and H2 as before are used, but with no catalyst so that the reaction does not take place.